Important Safety Information
CONTRAINDICATIONS: BRIUMVI is contraindicated in patients with:
- Active HBV infection
- A history of life-threatening infusion reaction to BRIUMVI
WARNINGS AND PRECAUTIONS
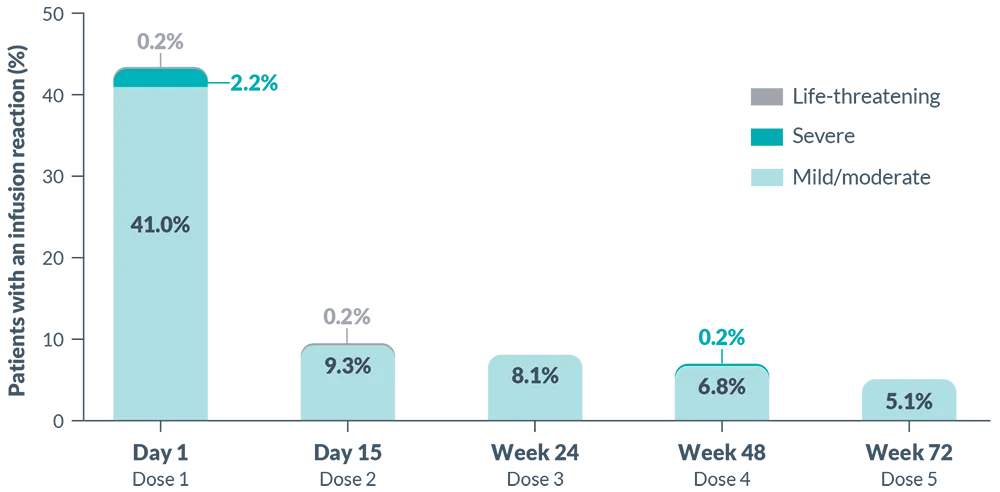
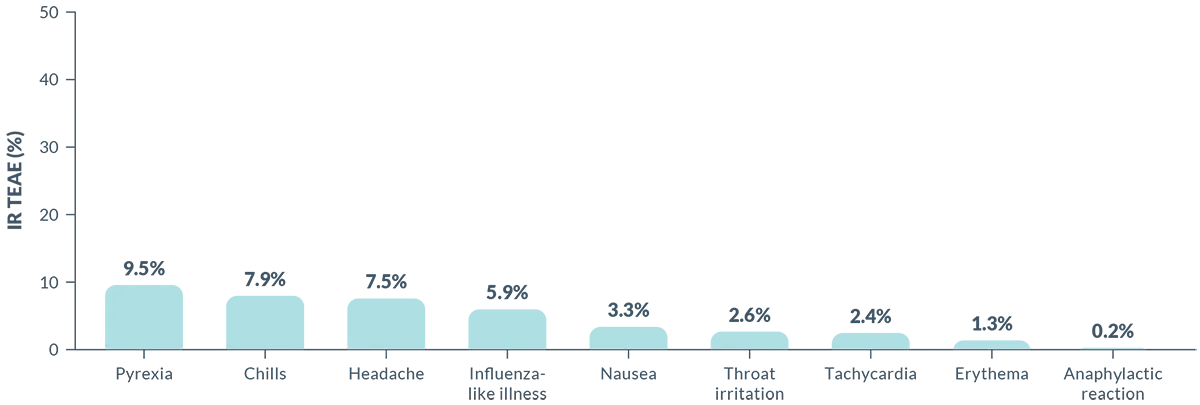
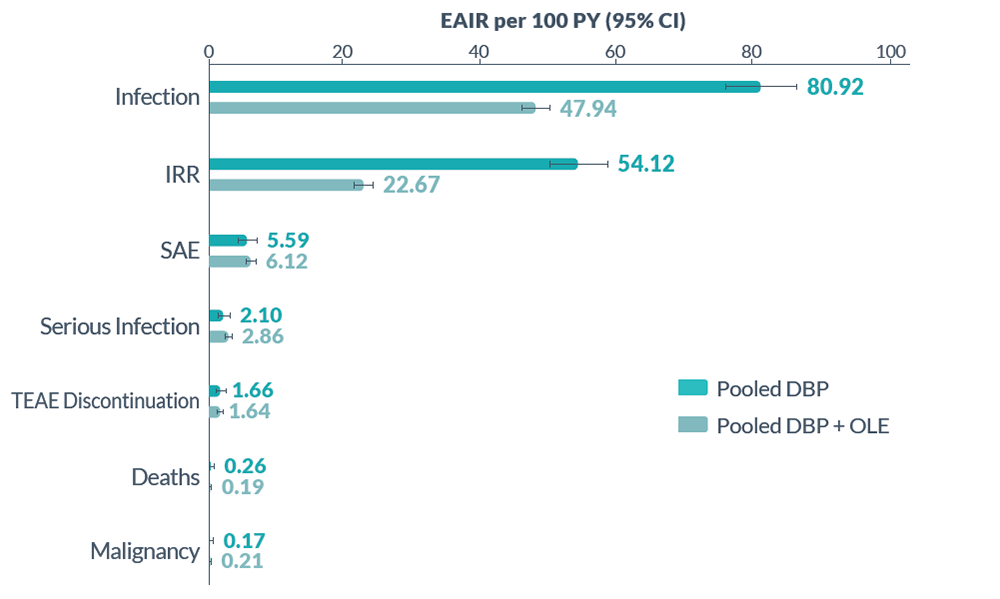
Infusion Reactions: BRIUMVI can cause infusion reactions, which can include pyrexia, chills, headache, influenza-like illness, tachycardia, nausea, throat irritation, erythema, and an anaphylactic reaction. In MS clinical trials, the incidence of infusion reactions in BRIUMVI-treated patients who received infusion reaction-limiting premedication prior to each infusion was 48%, with the highest incidence within 24 hours of the first infusion. 0.6% of BRIUMVI-treated patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe treated patients for infusion reactions during the infusion and for at least one hour after the completion of the first two infusions unless infusion reaction and/or hypersensitivity has been observed in association with the current or any prior infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer the recommended pre-medication to reduce the frequency and severity of infusion reactions. If life-threatening, stop the infusion immediately, permanently discontinue BRIUMVI, and administer appropriate supportive treatment. Less severe infusion reactions may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections: Serious, life-threatening or fatal, bacterial and viral infections have been reported in BRIUMVI-treated patients. In MS clinical trials, the overall rate of infections in BRIUMVI-treated patients was 56% compared to 54% in teriflunomide-treated patients. The rate of serious infections was 5% compared to 3% respectively. There were 3 infection-related deaths in BRIUMVI-treated patients. The most common infections in BRIUMVI-treated patients included upper respiratory tract infection (45%) and urinary tract infection (10%). Delay BRIUMVI administration in patients with an active infection until the infection is resolved.
Consider the potential for increased immunosuppressive effects when initiating BRIUMVI after immunosuppressive therapy or initiating an immunosuppressive therapy after BRIUMVI.
Hepatitis B Virus (HBV) Reactivation: HBV reactivation occurred in an MS patient treated with BRIUMVI in clinical trials. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with BRIUMVI. Do not start treatment with BRIUMVI in patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult a liver disease expert before starting and during treatment.
Progressive Multifocal Leukoencephalopathy (PML): Although no cases of PML have occurred in BRIUMVI-treated MS patients, JCV infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies.
If PML is suspected, withhold BRIUMVI and perform an appropriate diagnostic evaluation. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
MRI findings may be apparent before clinical signs or symptoms; monitoring for signs consistent with PML may be useful. Further investigate suspicious findings to allow for an early diagnosis of PML, if present. Following discontinuation of another MS medication associated with PML, lower PML-related mortality and morbidity have been reported in patients who were initially asymptomatic at diagnosis compared to patients who had characteristic clinical signs and symptoms at diagnosis.
If PML is confirmed, treatment with BRIUMVI should be discontinued.
Vaccinations: Administer all immunizations according to immunization guidelines: for live or live-attenuated vaccines at least 4 weeks and, whenever possible at least 2 weeks prior to initiation of BRIUMVI for non-live vaccines. BRIUMVI may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines during or following administration of BRIUMVI has not been studied. Vaccination with live virus vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with BRIUMVI During Pregnancy: In infants of mothers exposed to BRIUMVI during pregnancy, assess B-cell counts prior to administration of live or live-attenuated vaccines as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines. Inactivated or non-live vaccines may be administered prior to B-cell recovery. Assessment of vaccine immune responses, including consultation with a qualified specialist, should be considered to determine whether a protective immune response was mounted.
Fetal Risk: Based on data from animal studies, BRIUMVI may cause fetal harm when administered to a pregnant woman. Transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 B-cell depleting antibodies during pregnancy. Advise females of reproductive potential to use effective contraception during BRIUMVI treatment and for 6 months after the last dose.
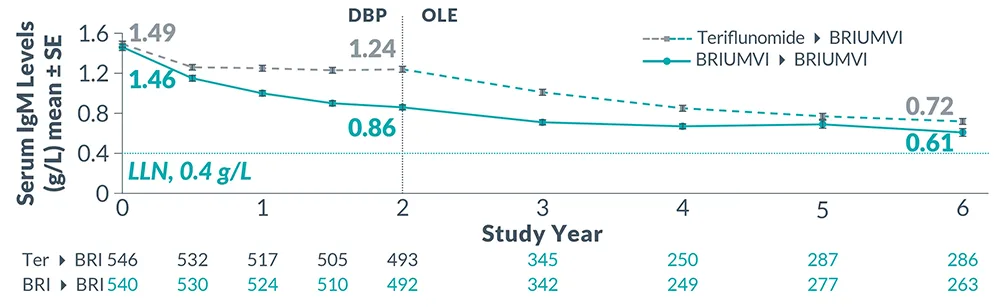
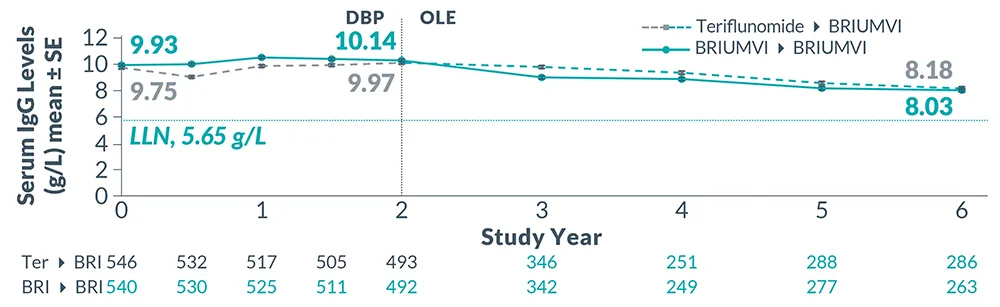
Reduction in Immunoglobulins: As expected with any B-cell depleting therapy, decreased immunoglobulin levels were observed. Decrease in immunoglobulin M (IgM) was reported in 0.6% of BRIUMVI-treated patients compared to none of the patients treated with teriflunomide in RMS clinical trials. Monitor the levels of quantitative serum immunoglobulins during treatment, especially in patients with opportunistic or recurrent infections, and after discontinuation of therapy until B-cell repletion. Consider discontinuing BRIUMVI therapy if a patient with low immunoglobulins develops a serious opportunistic infection or recurrent infections, or if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Liver Injury: Clinically significant liver injury, without findings of viral hepatitis, has been reported in the postmarketing setting in patients treated with anti-CD20 B-cell depleting therapies approved for the treatment of MS, including BRIUMVI. Signs of liver injury, including markedly elevated serum hepatic enzymes with elevated total bilirubin, have occurred from weeks to months after administration.
Patients treated with BRIUMVI found to have an alanine aminotransaminase (ALT) or aspartate aminotransferase (AST) greater than 3x the upper limit of normal (ULN) with serum total bilirubin greater than 2x ULN are potentially at risk for severe drug-induced liver injury.
Obtain liver function tests prior to initiating treatment with BRIUMVI, and monitor for signs and symptoms of any hepatic injury during treatment. Measure serum aminotransferases, alkaline phosphatase, and bilirubin levels promptly in patients who report symptoms that may indicate liver injury, including new or worsening fatigue, anorexia, nausea, vomiting, right upper abdominal discomfort, dark urine, or jaundice. If liver injury is present and an alternative etiology is not identified, discontinue BRIUMVI.
Most Common Adverse Reactions: The most common adverse reactions in RMS trials (incidence of at least 10%) were infusion reactions and upper respiratory tract infections.
INDICATION
BRIUMVI is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.